[ad_1]

An artistic rendition of an iVAX vaccine being prepared in the field.Credit: Justin Muir
In January, the US Food and Drug Administration granted breakthrough status to a vaccine for bacterial pneumococcal disease.
Developed by Vaxcyte in San Carlos, California, VAX-24 induces an immune reaction to Streptococcus pneumoniae by exposing the body to bacterial sugars linked to a carrier protein — known as a protein conjugate vaccine. Market-leading PREVNAR-20, produced by US pharmaceutical giant Pfizer, has a similar design. But whereas PREVNAR-20’s protein conjugates are purified from bacteria, those in VAX-24 are built biochemically.
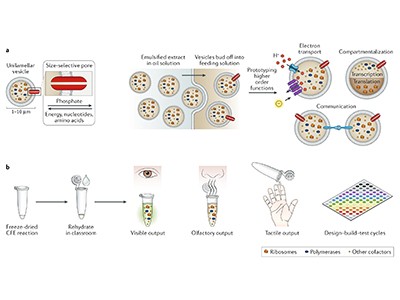
Vaxcyte is one of a growing number of biomanufacturing firms embracing this cell-free biosynthesis strategy. Instead of relying on yeast or bacteria to make biomolecules, the approach strips away all the components of a cell that make it ‘alive’ — the long strings of DNA, the complex origami of the endoplasmic reticulum and even the bulwark of the cell membrane — and freeze-dries what remains. Scientists can then rehydrate the dried material with water and add nucleic acids to program the molecular machinery to make an infinite array of proteins on demand.
Tobias Erb, a microbial physiologist at the Max Planck Institute for Terrestrial Microbiology in Marburg, Germany, likens the process to using a commercial cake mix: just add water and bake. To Michael Jewett, a chemical and biological engineer at Northwestern University in Evanston, Illinois, the strategy is more like popping open the bonnet of a car, removing the engine and repurposing it to power a drill.
“It allows biology to become more chemically diverse, and at the same time, it allows chemistry to become more complex,” Erb says. Jewett has shown, for instance, that the ribosomes in cell-free systems can be used to build biopolymers with new chemical backbones, as well as proteins. Other researchers have used cell-free systems to synthesize proteins using non-standard amino acids.
Cell-free gene expression
Researchers have been using cell-free systems for biochemical reactions for decades — but mostly in the research laboratory. Now, thanks to advances in both reliability and scale, cell-free synthesis is emerging as a major tool for everything from diagnostic-sensor development to vaccine biomanufacturing.
Still, challenges remain. The stripped-down simplicity that makes cell-free systems so desirable also complicates key protein modifications, including the attachment of carbohydrates (glycosylation) and folding assisted by molecular chaperones. Those hurdles will need to be overcome before cell-free technologies can be used for biopharmaceutical manufacturing and other applications.
But doing so, says Matthew DeLisa, a bioengineer at Cornell University in Ithaca, New York, is really just an issue of engineering. “Cell-free systems,” he says, “turn protein synthesis into more of a chemistry problem than a biology problem.”
Beer to biopharmaceuticals
From the natural fermentation processes that produce cheese and bread, to bioengineered cells that can pump out insulin, people have long used cells as living factories.
But efficient processes require careful balance — bioengineers must compete against the cell’s internal machinery, which is more interested in helping the cell to grow and divide than in doing industrially useful work.
“Cells don’t really want to make sustainable products. They don’t want to make insulin for us, they don’t want to act as a diagnostic. From an evolutionary perspective, that just hasn’t been their main objective,” says Jewett.

Michael Jewett works on cell-free processes at Northwestern University.Credit: Courtesy of Northwestern University
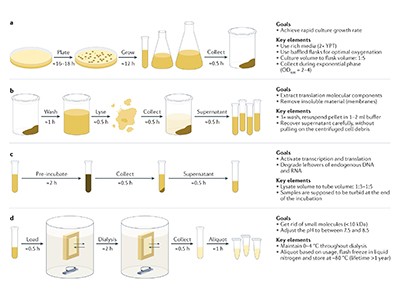
To make the process more efficient, scientists break open (or lyse) living cells to extract the molecular machinery of interest, leaving other components behind. In 1907, German chemist Eduard Buchner won the Nobel Prize in Chemistry for his work using cell-free yeast lysates to demonstrate the biochemistry of fermentation. Around half a century later, researchers at the US National Institutes of Health used bacterial cell-free extracts to decipher the genetic code.
“You have all the benefits of working with a living system without all the problematic overhead,” says Elizabeth Strychalski, who leads the Cellular Engineering Group at the US National Institute of Standards and Technology in Gaithersburg, Maryland.
So why has biomanufacturing been slow to adopt the technology? In part, it is simply down to inertia. “It’s just taken a long time for people to wrap their heads around the idea that we don’t need a living organism to do complex metabolism, to make complex molecular rearrangements, even to make complex proteins,” says James Swartz, a chemical engineer at Stanford University in California.
Proteins on demand
One reason for the slow transition is that it has been cheaper and easier to grow large quantities of bacteria and yeast than to run biochemical reactions, which require expensive reagents, energy sources and other materials. Cells also carry out those reactions in a defined order, something that is difficult to ensure in a vial. And they can generate higher yields of the desired product.
But now, years of molecular tinkering have made cell-free systems more efficient and cost-effective. The biggest factor behind industry’s growing interest, says Michael Nemzek, chief executive of Tierra Biosciences in San Leandro, California, is improvements in peripheral technologies such as long-DNA synthesis and miniaturization that make it easier to make longer proteins, and to test them in small batches.
The other key advance, Jewett says, involves storage. Instead of requiring refrigeration and a complex cold chain, researchers can store most cell-free preparations indefinitely and rehydrate them as needed. “It’s kind of like freeze-dried ice cream — something you can transport wherever you go,” he says. This gives the technology added speed and flexibility — features that are all too often missing from the global vaccine supply chain.

Cell-free processes could be used to make biosensors that detect contaminants in water.Credit: Northwestern University
Despite rapid advances in messenger RNA technology, most of the world’s vaccines are made of protein conjugates, which use a polysaccharide–protein duo to elicit an immune response. Theoretically, says Jewett, a lab could rehydrate a freeze-dried cell extract and begin producing such vaccines in an hour. Public-health officials wouldn’t have to wait for pharmaceutical companies to ship doses from halfway around the world, says Stanford bioengineer Jessica Stark. Pharmacies and health departments could make vaccines and therapeutics on demand.
“The COVID-19 pandemic has really shone a light on the fact that we need better ways to make and distribute medicines very quickly in order to address emerging pathogens,” Stark says.
Cell-free gene expression: an expanded repertoire of applications
Vaxcyte’s partner, Sutro Biopharma in South San Francisco, California, relies on cell-free biosynthesis to design, discover and synthesize protein therapeutics for cancer, including antibodies that deliver drugs to specific cells or that recognize two antigens instead of just one, as well as immune-system proteins called cytokines. Tierra Biosciences uses cell-free biosynthesis to create everything from custom proteins for drug discovery and clinical trials to peptide libraries for screening and research. “You can upload a protein sequence, wait a few weeks, and we’ll FedEx you a package,” Nemzek says.
Khalid Alam, chief executive of Stemloop in Evanston, Illinois, has taken his company in a different direction. Instead of drugs, the firm uses cell-free systems to create biosensors from DNA-binding proteins called transcription factors. These proteins are switched on by molecules in the environment to induce the expression of a fluorescent or colour-coded protein that the user can see. Alam hopes for a wide range of applications, from treating infectious diseases to detecting heavy metals in drinking water — what Jewell likens to “a pregnancy test for water”.
“Our mission is to unlock this power that biology has to sense and respond to the changing conditions of the environment,” Alam says. The sensors “empower anybody to sense the world around them”.
Moving forward
Drawn by the possibility of building therapeutics and biosensors on demand, the US Army awarded US$13 million last March to fund a new Cell-Free Biomanufacturing Institute at Northwestern University, with Jewett at the helm. Its goal is to tackle ongoing issues of cost, yield and reproducibility to facilitate more widespread use of cell-free systems.
Antibodies, for instance, have become the bread and butter for many pharmaceutical companies, and DeLisa says that the ability to synthesize these molecules using cell-free methods would be a “game changer”. But even the simplest antibody remains too complex for existing cell-free platforms to tackle, he says. There’s no fundamental reason that the technology can’t synthesize an antibody, but scientists have yet to work out precisely how to do so.
NatureTech hub
Part of the problem is folding: many complex proteins require other proteins called chaperones to attain their final form.
There’s also the sticky issue of glycosylation. Half of all human proteins are tagged with carbohydrate groups that control their activity. Cell-free systems from bacteria lack the machinery to add sugars to proteins, whereas mammalian cell extracts can affix both desired and undesired chemical modifications. DeLisa and others are developing specific glycosylation modules for bacterial cell-free extracts that would allow researchers to maintain control over the process and keep it precise.
Another obstacle, says Strychalski, is the lack of tools to make cell-free reactions run more like computer code than alchemy. She, for example, is working on refined probes that would allow researchers to follow reactions at the molecular level rather than just indicating success at the end.
Even if these obstacles are overcome, cell-free synthesis will probably never completely replace its cell-based counterpart, Strychalski says. Some companies have already optimized their production facilities to work with cells, and some molecules might always be easier and cheaper to make that way.
For all their apparent simplicity, cell-free systems remain enormously complex. Researchers don’t yet have a good enough handle on what’s happening in the test tube to model and scale it up effectively. But as cell-free systems take their place in the biomanufacturing toolset, they are providing researchers with a new and increasingly attractive option: just add water.
[ad_2]
Source link