Wagner, M., Hendy, I. L., & Lai, B. (2022). Characterizing Ag uptake and storage in the marine diatom Thalassiosira pseudonana: Implications for Ag biogeochemical cycling. Marine Chemistry, 247, 104175. https://doi.org/10.1016/j.marchem.2022.104175
Silver in the ocean
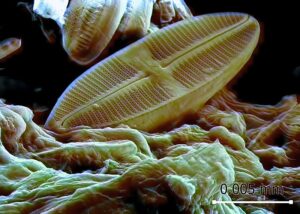
The ocean is made up of more than just water with some salt. Within the salty sea there is every element from oxygen to iron to einsteinium (yes, there is an element named after Einstein), just in different amounts. One element that is not commonly associated with the ocean, but instead jewelry stores, is silver. There might not be much silver in the ocean, but it can be used by some marine creatures. While corals and clams use calcium (in calcium carbonate) to form their hard exteriors, minute organisms known as diatoms (types of phytoplankton that are free floating photosynthesizers) use silicon to form their shells in a similar form to quartz. Silver is chemically similar to silicon, so organisms, like diatoms, can take it up into their bodies in place of silicon. Silver can act like silicon and an organism with a single cell, like a diatom, can’t tell the difference. Much like your cells can’t tell the difference between radium and calcium, which was an issue back when radium was in everything from paint to face creams and radium could get into your bones in the place of calcium.
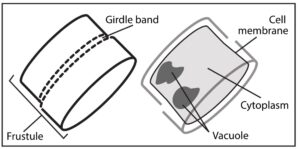
Diatoms recreating the past
Just like paleontology deals with fossils of plants and animals, paleoceanography works to reconstruct what the ocean looked like from a hundred years ago to millions of years ago. Diatoms using silver to make their shells instead of silicon can help scientists recreate the past of the ocean. By looking at the amount of silver in the sediment at the bottom of the ocean, scientists can determine how abundant diatoms and the ocean were at a given period of time. The deeper you dig in the bottom of the ocean, the further back in time you go and the more you can discover about the past of the oceans. One issue that has snagged scientists in the past when using silver to understand how productive diatoms were, is how the diatoms store silver. Many scientists argued that diatoms store silver in their exterior, while others argued for the interior. While this may not seem like an important distinction, it matters when it comes to how the diatom breaks down after it dies. Anything on the outside of the diatom is going to be broken down first, before anything on the inside. A team of scientists lead by Dr. Meghan Wagner set out to understand where diatoms store their silver.
How silver (Ag) is stored in diatoms
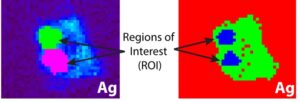
The scientists, based out of University of Michigan and Argonne National Laboratory, used a device known as a synchrotron micro-XRF microprobe. Essentially it is a small device that allows scientists to see where different elements are being kept within a cell. The team found hotspots of silver within the diatom. If silver was kept in the outside skeleton, the scientists would have expected it to be all around the diatom. Instead, they found that the silver is mostly being kept within the vacuoles of the diatom with only small amounts being held in the exterior skeleton. The vacuoles hold water for the cell and help to maintain the right pressure inside the diatom compared to the outside pressure. Diatoms holding silver in their squishy middles allows for the silver to be more easily scavenged when it dies.
Understanding the past to protect the future
Since other organisms can quickly use up some silver that is released, silver can’t yet be used to tell exactly how may diatoms used to be in an area. But it can help scientists to determine where diatom hotspots used to be. Areas that have high amounts of silver in the sediment can be linked to large concentrations of diatoms. Diatoms are responsible for about 40% of all of the photosynthesis in the ocean, making them important players in the oxygen production of the world. Understanding how this minuscule creatures function and where they like to congregate will help scientists understand how the changing climate will impact not only diatoms, but the ocean as a whole.

While I have never lived close to the ocean (Minnesota, Arizona, and now Central New York), it has always had a special place in my heart. I’m currently a PhD candidate at the State University of New York College of Environmental Science and Forestry (SUNY-ESF) studying reef and coastal biogeochemistry. I focus on the lipid and trace metal composition of settling particles and surface sediment in coastal systems, primarily studying coral reefs. When not diving or in the lab you can find me hiking with my dogs, reading, cross stitching, or just enjoying a good cup of tea!

