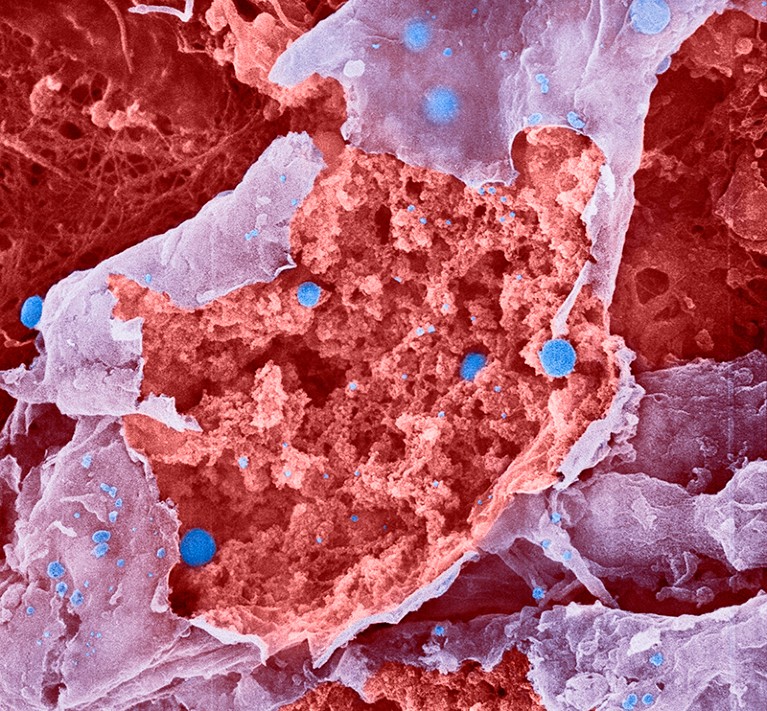
Particles of influenza virus (blue; artificially coloured) bud from a burst cell.Credit: Lennart Nilsson, Boehringer Ingelheim International GmbH, TT/SPL
A case of influenza can make even the toughest people take to their beds and lose their appetites. Now, scientists have identified neurons in mice that notify the brain of a flu infection, triggering decreases in movement, hunger and thirst1.
Similar neurons connecting to other parts of the body might notify the brain of other infections, too, the authors say. The work was published on 8 March in Nature.
“This study flips previous thinking on its head,” says Ishmail Abdus-Saboor, a sensory biologist at Columbia University in New York City who was not involved in the research. “This is paradigm-shifting in terms of how we think about sickness behaviour.”
Brain surveillance
Before this research, “it was not clear how the brain becomes aware that there’s an infection in the body”, says study co-author Stephen Liberles, a neuroscientist at Harvard Medical School in Boston, Massachusetts. Scientists generally thought that messenger molecules from the site of infection move through the bloodstream to the brain, diffusing into it to directly activate the regions that kickstart the sickness-behaviour program.
Among the top candidates for these messenger molecules were signalling chemicals called prostaglandins, which are made in infected tissues. Aspirin and ibuprofen block prostaglandin production — and also suppress sickness behaviours, hinting that prostaglandins are key to triggering such behaviours.
Guardians of the brain: how a special immune system protects our grey matter
The authors showed that a specific prostaglandin receptor, called EP3, is responsible for generating sickness behaviours. EP3 is found on neurons throughout the body, including the brain. To test its function, the researchers deleted the brain’s EP3 receptors in mice and infected the animals with flu virus. The mice still changed their behaviour — indicating that the brain is not getting infection dispatches from blood-borne prostaglandins.
Instead, the authors found that the key agents are a specific EP3-containing population of neurons located in the mouse’s neck. These neurons have branches that stretch from the mouse equivalent of the tonsils to the brainstem. This geography makes sense: the tonsil area “serves as the interface between the outside air and what goes in the airway”, says study co-author Na-Ryum Bin, a neurobiologist also at Harvard. The area is rich in immune cells that churn out prostaglandins when they encounter pathogens.
The results tell a narrative of illness: flu viruses enter the airway and infect throat cells, triggering prostaglandin production, and these previously unappreciated neurons respond. The infection alert then travels along the neurons’ branches on “a dedicated highway to the brain”, Abdus-Saboor says.
A highway to the brain
Neural pathways do something blood-borne signals cannot: they give the brain information about exactly where the infection is occurring. The authors note that many other types of neuron have receptors for prostaglandins and other immune-related signals. They suggest that further dedicated pathways could exist, including ones for detecting gut infections, triggering nausea.
Flu and colds are back with a vengeance — why now?
The study also revealed a paradox. Scientists assume that there is an evolutionary advantage to sickness behaviour. But when the team blocked those behaviours, such as food avoidance, mice were less likely to die of the flu. Liberles speculates that this behaviour-modifying system might have evolved because it is beneficial in most cases of infection — even if it isn’t in all. Alternatively, behaviours such as immobility might be advantageous by reducing the spread of pathogens through populations.
The new results don’t tell the full story. The infection-sensing tonsil neurons are responsible for sickness behaviour only during a flu infection’s first stage, which affects the upper airway and lasts roughly a week. As the virus moves into the lower respiratory tract over the course of the illness, another nerve pathway takes over the job of driving sickness behaviours. “If we could find a way to block that second pathway, that, in combination, could have tremendous clinical impact,” Liberles says.

