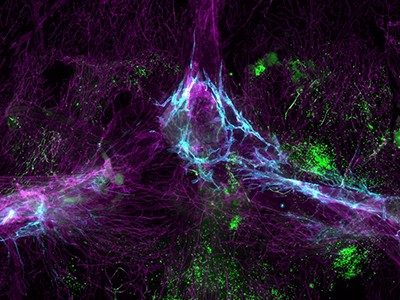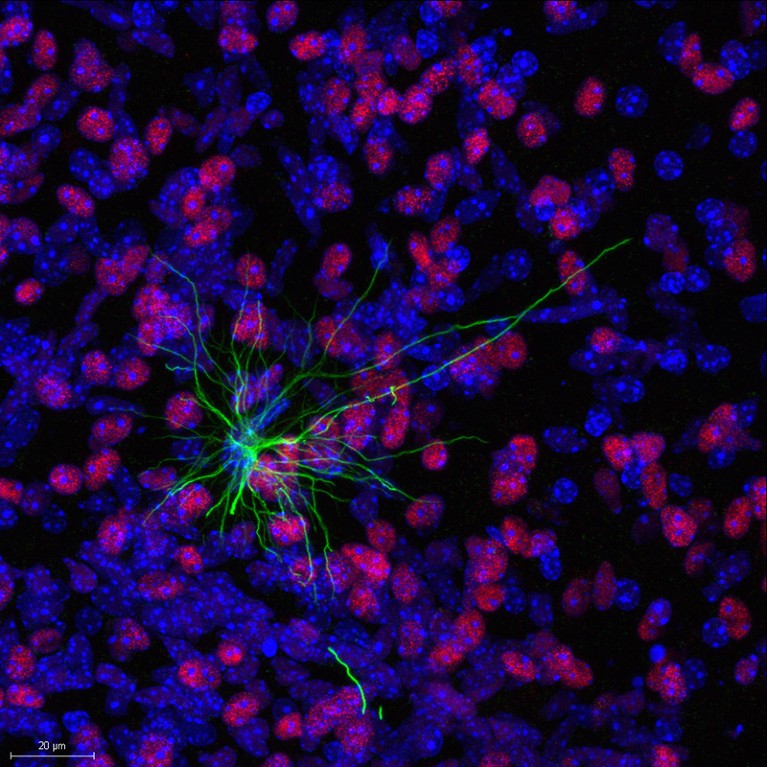
A mouse’s brain (red and blue) hosts a human astrocyte (green) that arose from transplanted neural stem cells.Credit: Liu et al./Cell (2023)
In a technical “tour de force”, researchers have analysed multiple traits of individual cells to pinpoint those that give rise to crucial components of the human brain.
The analysis, published on 16 March in Cell1, uses a combination of protein and RNA analysis to painstakingly purify and classify individual stem cells and their close relatives isolated from human brains. Researchers then injected different types of cell into mice and monitored the cells as they divided and their progeny took on specialized roles in the brain.
The hope is that this study, and others like it, will illuminate how such developmental programmes go awry in neurological diseases — and how they can be harnessed to create new therapies. “The census of stem and progenitor cells in the developing human brain is really just beginning,” says Arnold Kriegstein, a developmental neuroscientist at the University of California, San Francisco, who was not involved in the research. “This work offers a nice window into some of that complexity.”
Cellular ensemble
The brain is an intricate symphony of different cells, each of which performs essential functions. Star-shaped cells known as astrocytes, for example, are important for supporting metabolism in neurons, and loss of astrocyte function is linked to neurodegenerative conditions such as Alzheimer’s disease. Oligodendrocytes are cells that create a protective, insulating sheath around the connections between neurons. When they are damaged — as in diseases such as multiple sclerosis — communication between neurons slows or stops altogether.
Guardians of the brain: how a special immune system protects our grey matter
To understand how such cells arise, stem cell biologists Irving Weissman and Daniel Liu and their colleauges at Stanford University in California harnessed new technology that would allow them to study the developmental destiny of individual cells taken from human brains.
The team isolated brain cells from human fetuses that were 17–19 weeks old and tested the cells for a battery of 11 proteins on the cell surface, including six that are associated with neural cell development. They also analysed RNA levels as a measure of gene activity and used this information to purify 10 kinds of cell that are likely to give rise to astrocytes, oligodendrocytes and neurons.
The researchers then injected the purified cells into mouse brains. Six months later, they analysed those mice to find out where the cells and their descendants had migrated, and what cellular identities they had taken.
The approach enabled the team to define a new kind of progenitor cell that gives rise to glial cells, a grouping that includes astrocytes and oligodendrocytes (see ‘How to make a brain’). These progenitors are derived from cells that are more sparse in mouse brains than in human brains, says Liu. “We think that this cell type might be important for specific adaptations that primate brains have made,” he says.
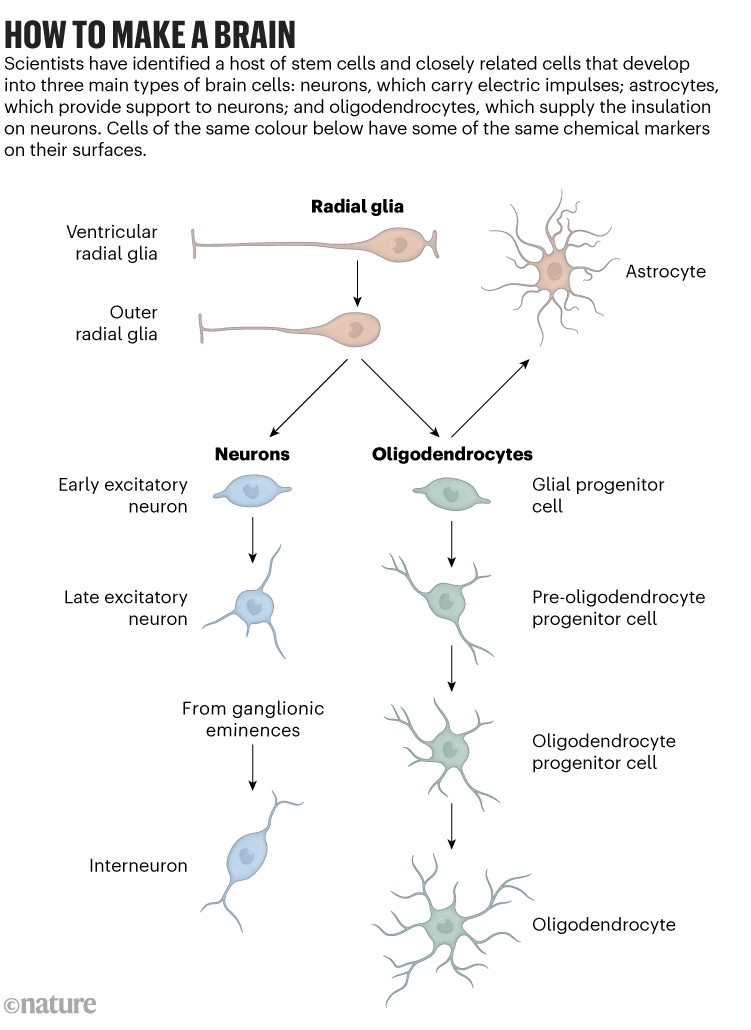
Source: Ref 1.
The team also found that high levels of a protein called Thy1 are associated with cells in the oligodendrocyte lineage. This runs counter to previous findings, which suggested that Thy1 was a marker for neurons rather than oligodendrocytes, says Steven Goldman, a neurologist at the University of Rochester Medical Center in New York and head of central nervous system therapy at Sana Biotechnology in Cambridge, Massachusetts.
Such differences could also be the result of the new approach’s improved resolution of different cell types, Goldman says, adding that the work is “a technical tour de force. … They upped the level of the field.” Weissman says that the technique might be useful for studying other kinds of stem cell as well.
Complex brew for a brain
The study is an important contribution to the growing knowledge of the cellular lineages that give rise to the human brain, agrees Kriegstein. But he notes that the development of human stem cells in mice might not fully reflect how the cells would develop in human brains.
Goldman worries that the lineages derived by the analysis do not reflect the plasticity of neural cell development. Other research has found that some cells in the brain can begin down one lineage, only to change paths and emerge as an unrelated neural cell, he says. Liu and Weissman think that some of that apparent plasticity was instead an artefact of studying mixtures of cells, and might not be as pronounced when using the stringent selection criteria for purifying cells that they developed.
But Goldman suspects that other factors influence how committed neural cells remain to their lineage. “The nervous system is more complicated in terms of diversification,” says Goldman. “There’s still a lot to learn.”