THE PAPER IN BRIEF
• Genetically encoded calcium indicators (GECIs) are designer proteins that react to intracellular calcium-ion (Ca2+)influxes — which occur during the activation of neurons — by emitting light.
• GECIs have enabled researchers to gain myriad insights into the workings of the brain, but until now the proteins have lacked the precision to record a fundamental unit of activity, known as the action potential, in single neurons.
• Writing in Nature, Zhang et al.1 describe a GECI that marks a step towards solving this problem.
MICHAEL B. RYAN & ANNE K. CHURCHLAND: Lamps that illuminated the brain
Imagine rowing across still, dark waters on a summer night, when the water is suddenly transformed by the brilliant glow of countless jellyfish illuminating a hidden world of activity. In the 1960s, scientists from the Friday Harbor Laboratories in Washington collected nearly 10,000 specimens of Aequorea jellyfish by hand, with the goal of isolating the organic component responsible for this natural bioluminescence2. The scientists extracted and purified a calcium-sensitive protein that they named aequorin, together with an associated green fluorescent protein (GFP). Soon after, researchers loaded purified aequorin into living cells to track changes in Ca2+, an ion that is central to neuronal activity3. This ability to measure neuronal activity by proxy — through flashes of light — revolutionized the field of neuroscience, and led to the identification of previously unknown dynamics in the brain.
By injecting aequorin into neurons, scientists discovered how changes in the levels of intracellular Ca2+, resulting from neuronal activation, contribute to the process by which these cells release neurotransmitters to communicate with one another4. Several decades after aequorin was first isolated, another breakthrough came in the form of GECIs. Whereas aequorin and other calcium-sensitive dyes require manual loading into cells, GECIs are engineered to become part of the organism’s DNA5,6. The most widely used GECIs, known as the GCaMP family7, consist of a calcium-binding protein called calmodulin and a bound peptide (a short string of amino acids), fused to a modified form of GFP. When Ca2+ binds to calmodulin, this triggers a conformational change in the fusion protein, which causes GFP to emit light (Fig. 1a).
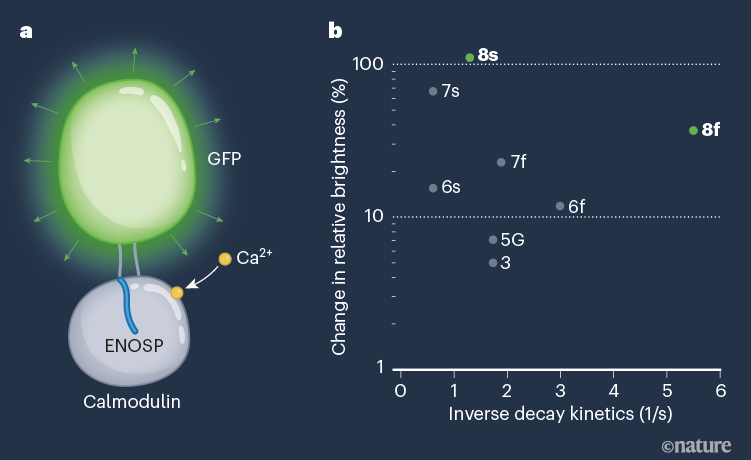
Figure 1 | Structure and response profile of GCaMP8 proteins. a, Zhang et al.1 have developed GCaMP8 — optimized forms of genetically engineered calcium indicators, which respond to intracellular changes in the levels of calcium ions (Ca2+) by fluorescing. GCaMP8 proteins consist of a transmembrane calcium-binding domain (calmodulin), bound to a peptide (a fragment of endothelial nitric oxide synthase; ENOSP) that modifies the activity of calmodulin. Two linkers attach calmodulin to a modified form of green fluorescent protein (GFP). When Ca2+ binds, a change in protein configuration leads to GFP fluorescence. b, The GCaMP8 sensors (8s and 8f) show several improved qualities compared with existing GCaMP sensors, including higher sensitivity (shown as a change in relative brightness from the protein’s baseline) and faster decay kinetics (a key parameter in response rate, shown as an inverse scale in 1/seconds).
Despite the potential of these calcium sensors, early versions of GCaMP were hampered by issues such as poor response kinetics, low calcium sensitivity and limited correlation with the electrical activity of neurons. Initial versions of the sensors could detect only large changes in Ca2+ brought about by tens of action potentials, with response kinetics on the order of several hundred milliseconds.
Considerable effort has since been dedicated to improving the performance of GCaMP sensors through structure-guided design. Later versions of GCaMP have even been optimized for sensitivity or speed, with ‘slow’ variants maximizing signal strength, and ‘fast’ variants maximizing response kinetics. Fast variants of GCaMP can now detect changes in calcium in time frames of tens of milliseconds. Other GECIs have also been developed that emit different wavelengths of light in response to Ca2+, enabling several populations of neurons to be imaged simultaneously.
Read the paper: Fast and sensitive GCaMP calcium indicators for imaging neural populations
These advances have enabled scientists to capitalize on the versatility, specificity and longevity of GECIs. The fact that they are genetically encoded allows them to be expressed continuously in many cells at once. This has facilitated in vivo measurements of large populations of cells in flies, rodents and primates. The cell type in which GECIs are expressed can also be controlled genetically, which has helped researchers to understand the variability of neural circuits. For instance, GECIs have been used to delineate subpopulations of neurons in the brain’s cortex that have distinct calcium dynamics and roles in decision-making8. They have also been used to measure Ca2+ in non-neuronal cells in the brain. For example, calcium imaging of astrocytes — the most abundant non-neuronal cell type in the brain — has revealed how neurotransmitters such as dopamine shape the response of astrocytes to neural activity9,10.
Nonetheless, the latest GCaMP iterations have fallen short of reliably identifying individual action potentials under the ‘noisy’ conditions in living brains. Several research groups have been racing to identify GCaMP variants that can detect these changes — which occur over just a few milliseconds — without sacrificing brightness. Zhang and colleagues’ work introduces a competitor to this race, and points to a bright future for calcium imaging.
YIYANG GONG & CASEY BAKER: Time for the next generation
Single action potentials are the fundamental unit of neural communication. Zhang and colleagues’ development is therefore a major advance. The authors’ GCaMP8 sensors have significantly faster kinetics than do existing start-of-the-art GECIs (Fig. 1b), such as the GCaMP7 and XCaMP series11,12. The GCaMP8 sensors also avoid the typical trade-off between sensitivity and speed, whereby slower sensor kinetics produce larger optical responses.
Zhang and colleagues based their sensors on GCaMP6. The authors optimized this protein by engineering different versions of several of its modules, including the two linkers that connect GFP to the bound peptide and to the calmodulin domain that grabs the peptide when Ca2+ binds. They paid particular attention to the interface between the bound peptide (which in GCaMP8 is a fragment of endothelial nitric oxide synthase) and the calmodulin domain, because this region of the sensor has a crucial role in its response and kinetics12,13. The team then used an optimization process that involved replacing many amino acids and performing several rounds of testing to identify the best-performing sensors. They validated the performance of these indicators in flies and mice. The strong performance of the GCaMP8 sensors suggests that the way in which Ca2+ induces fluorescence in this series is different from that in other GCaMPs.
A breakthrough method that became vital to neuroscience
The fast kinetics and high accuracy of the GCaMP8 sensors will enable researchers to analyse phenomena in live animals that could previously have been studied only by using electrical measurements or genetically encoded voltage indicators (GEVIs), which respond to changes in voltage by fluorescing (much like how GECIs respond to calcium). Until now, it has been assumed that GECIs and GEVIs report different types of neural activity — GEVIs determine the timing of action potentials, and GECIs reveal key activity in neuronal compartments (for example, by measuring Ca2+ dynamics in processes called dendrites that receive signals from other neurons)14.
However, with the development of GCaMP8, the information that can be gained from these tools has converged. Zhang and colleagues show, through computational analyses of the light bursts released by GCaMP8, that their sensors can detect action potentials nearly as accurately as can voltage imaging15,16. Similarly, GCaMP8 could reliably detect when fly neurons that normally fire frequently become transiently inactive, providing similar results to GEVI-based measurements17,18.
When both voltage and calcium imaging can be used to record from many neurons simultaneously, practical experimental considerations might persuade future researchers to choose calcium imaging over voltage imaging. GCaMP8 is compatible with existing microscopy configurations and preparation methods. The series can be used to examine the activity of broad neuronal networks on a larger scale than is possible using voltage imaging. One might imagine that the GCaMP8 series will soon be used to interpret detailed, millisecond-by-millisecond sequences of activity that flow through many neurons in a specific area of the brain.
The fast kinetics associated with this generation of GCaMPs, along with existing GEVIs, will motivate the development of rapid optical-microscopy and imaging technologies. Such microscopes would be able to detect transient bursts of light emitted from many neurons simultaneously. But for now, the fact that calcium-sensor kinetics is no longer the bottleneck in interpreting rapid neural activity will be welcomed by many neuroscientists.

