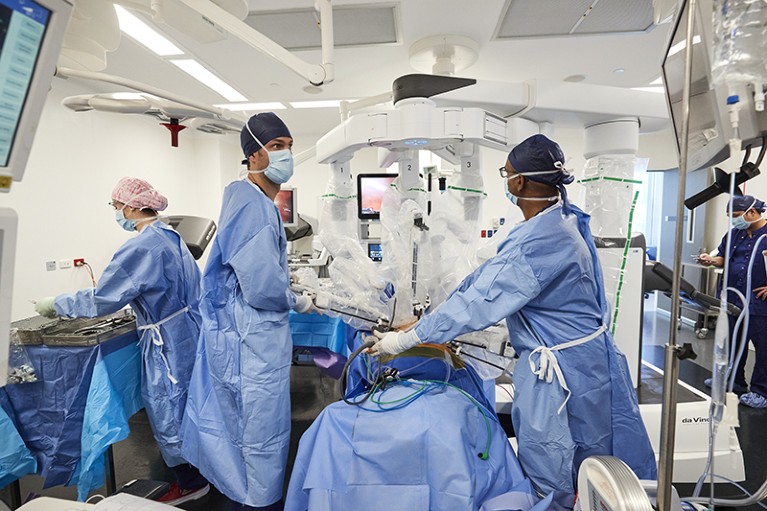
Ruban Thanigasalam (centre, right) has used a robotic surgical system for 15 years, and says it benefits patients and surgeons.Credit: Ken Leanfore
The COVID-19 pandemic has stretched health-care workforces around the world to their limits, as illness and burnout extract a toll from clinicians, nurses and staff. The need for innovations that can reduce workloads is pressing and has intensified interest in artificial intelligence (AI) and robotics as potential technologies to help in many ways, from processing doctors’ notes, to improving surgical outcomes, and even assisting clinicians with rapid decision-making during crises.
Cancer diagnosis and treatment have been especially affected by the pandemic, as hospital resources are diverted to urgent infectious-disease outbreaks, and health-care staff are ill or in isolation. Many of the key pressure points in this field are tasks that lend themselves to innovative solutions using AI and robotics. One of these is image processing for cancer screening and diagnosis; for example, checking mammograms. “Screening takes a lot of radiologists’ effort, and 98.5% to 99% of the mammograms are normal,” says Ioannis Sechopoulos, a medical imaging specialist at Radboud University Medical Center in Nijmegen, the Netherlands.
Nature Index 2022 AI and robotics
Sechopoulos and colleagues conducted a trial1 in which they compared an AI system with 101 radiologists in assessing 2,652 mammography images — 653 of which had already been found to be malignant. The AI had previously been trained using a database of more than 9,000 mammograms with cancer and 180,000 without. The trial found that the AI was as good as the average radiologist in detecting cancerous lesions, but less accurate than the best radiologists in the trial. But, Sechopoulos says, the aim of AI here would not be to remove the need for radiologists altogether, but to reduce their workload by acting as a second opinion in place of another human.
This approach is already being used in Copenhagen to help manage a backlog of breast-cancer image analysis that has built up owing to workforce shortages in the pandemic. Here, a system generates a risk score for the image — a high score indicating a high risk that a cancerous lesion is present. If the AI assesses an image as low risk, it is reviewed by only one human radiologist, whereas all other images are assessed by the usual two. Another approach that Sechopoulos and colleagues are exploring is to have the AI act as the second reader, and only if the AI and human radiologist disagree does the mammogram get analysed by a second person. Their unpublished results suggest no difference in the number of cancers detected while at the same time halving radiologists’ workload.
AI is also making inroads in the detection and diagnosis of skin cancer. A 2021 study2 used deep-learning technology to analyse an image of a large area of skin, such as a patient’s back, then categorized all the markings on the skin according to their level of suspicion so that high-risk lesions could be examined more closely by a dermatologist.
AI is also being applied to detecting cervical cancer and lung cancer. In these cases, as with breast and skin cancer, machine-learning algorithms learn to distinguish between malignant and benign lesions — or different types of malignant lesions — on imaging data sets, and then apply those learnings to help with screening and diagnosis.
When it comes to the treatment of cancer, robotics is already entrenched in the form of robotic-assisted surgery, particularly for cancers that are found in the pelvic region where space and manoeuvrability are limited. It is also helping to relieve pressure on overstrained hospitals and resources by reducing blood loss during surgery and reducing hospital stays after surgery. Urological surgeon Ruban Thanigasalam, from the Chris O’Brien Lifehouse cancer treatment centre in Sydney, Australia, and the University of Sydney, has been performing prostate-cancer operations using a robotic system for 15 years, and has seen the benefits for patients and surgeons. In robotic-assisted operations, the surgeon sits at a console in the operating room, remotely controlling the surgical instruments while being able to see what they’re operating on via a microscope. It’s minimally invasive, requires only small incisions to insert the operating instruments, and the translation of the surgeon’s wrist movements to the instruments allows for greater freedom in the tight space.
Studies comparing robotic surgery with laparoscopic and open procedures suggest they are equivalent in terms of cancer outcomes, but, says Thanigasalam, “if you’re looking at things like blood loss, length of hospital stay, and complications versus open surgery”, then using robotics is a “no brainer”.
He says the average length of stay for a robotic prostatectomy is shorter than for open surgery; a robot manufacturer-sponsored study3 published in 2014 found an average in-patient stay of 2.2 days for robotic surgery compared with 3.2 days for open surgery. Robotic surgery has made the greatest inroads in abdominal surgery, but Thanigasalam says it is also being explored for breast surgery. There is an economic barrier to accessing robotic surgery, because the instruments used by the robotic systems are replaced after just a few operations at a cost of many thousands of dollars each. But as the market opens up, Thanigasalam hopes the cost will fall and access will be increased.
Although there’s much excitement around AI and robotics in clinical medicine, there are also concerns that these new technologies come with significant risks.
Internal medicine specialist, Joann Elmore, at the David Geffen School of Medicine at the University of California, Los Angeles, hopes AI will better support medical practice but cautions that the despite a “tsunami” of algorithms being developed, “the actual evaluation of them after they’ve been implemented is sorely lacking”. For example, the outcomes of cancer diagnosis and treatment will not be truly known for many years, and Elmore questions whether AI-assisted diagnoses have been compared over those longer terms. There is also the risk that AI will flag lesions as cancerous even though they might not ultimately lead to ill health or premature death. “How do we know it wasn’t over-diagnosed?” she asks. AI is “very good at detecting things, so we’ll need to carefully modulate that threshold, so that the AI doesn’t worsen over-diagnosis”.
Anjali Mazumder, whose work at the Alan Turing Institute in London focuses on AI’s impact on social justice and human rights, says that AI technologies are also learning from data that are affected by human and structural biases as well as historical and cultural issues. For instance, that could mean that an AI learns to detect melanoma from a data set in which patients are predominantly white, so it may be less accurate at diagnosing skin cancer in patients with dark skin. Or an algorithm that helps guide diagnoses may have ‘learnt’ from human decision-making where systemic racism has led to Black patients being more likely to be underdiagnosed or misdiagnosed.
Mazumder says there is growing awareness of these risks in applying AI to medicine, but addressing them will require a multidisciplinary approach that involves health-care professionals, social scientists, anthropologists and communities in algorithm design. This will mean ensuring AI technologists across research institutes and industry “are working more closely with people from diverse disciplines to really consider how can we do this better, so we can avoid the potential pitfalls”.