A portable device that recycles the dialysate used in kidney dialysis is in development.Credit: Jeremy Barribeau
Richard Stacewicz knows how much dialysis alters a person’s life. He first underwent the procedure after his kidneys inexplicably failed in 1982, cutting short a motorcycle trip through the southwestern United States. He spent more than a year visiting a haemodialysis centre three times a week, for four hours at a time. At the centre, a machine the size of a small refrigerator removed toxins from his body and rebalanced his blood chemistry. The procedure kept him alive, but reduced his quality of life. “It was pretty miserable,” he says. “I couldn’t do very much for at least the next six or so hours after dialysis — I had to go home and lay down.” Worse, he couldn’t do the travelling he loved.
Eventually, Stacewicz was fortunate enough to receive a kidney transplant. He and his wife resumed their travels, crossing Eastern Europe on motorcycles in the late 1980s. But the transplant failed in 1994, and he spent another year sitting in chairs, attached to dialysis machines. In 1995, his sister donated a kidney, and that one lasted him until about three years ago, when it became cancerous and had to be removed. Since 2019, he’s been back on dialysis.
Part of Nature Outlook: Chronic kidney disease
The experience led Stacewicz, who recently retired from Oakton Community College near Chicago, Illinois, where he taught history and global issues, to join the Kidney Project, a research programme run by the University of California, San Francisco (UCSF), that’s working to develop an implantable bioartificial kidney. Stacewicz is on the project’s patient advisory council and seeks to raise awareness and financial support for further development and clinical trials.
The Kidney Project is one of a handful of programmes around the world that is attempting to replace conventional dialysis with portable or implantable artificial kidneys. The effort is boosted by the KidneyX prize, a collaboration between the US Department of Health and Human Services and the American Society of Nephrology, which provides money to jump-start innovation in the field.
Dialysis hasn’t fundamentally changed since the early 1960s. Portable or, ultimately, implantable devices could improve survival rates and quality of life. The chief challenges are how to replicate the activity of living kidneys as closely as possible, and how to do so without the huge volume of water used in conventional haemodialysis. The machines in dialysis centres, which filter blood through polymer membranes, weigh more than 100 kilograms and require 120–180 litres of water each session to flush away waste.
Such in-centre haemodialysis, which is what most people receive, is a poor substitute for living kidneys, says Shuvo Roy, a bioengineer at UCSF and technical director of the Kidney Project. “Our kidneys do seven or eight different functions,” he says. A dialysis machine provides just one of those functions — “but even then, doesn’t do it very well”, he adds. The large swings in blood chemistry that come from doing a rapid cleanse every couple of days, instead of the steady, constant filtering the kidneys perform, places stress on the body, notably the heart. The five-year survival rate of people on haemodialysis is below 50% — worse than some types of cancer.
The ultimate solution would be for everyone on dialysis to receive a new kidney, but there aren’t enough donor organs available for transplants. So, to create an implantable substitute that would replicate the kidney more closely, Roy and the medical director of the Kidney Project — William Fissell, a nephrologist at Vanderbilt University Medical Center in Nashville, Tennessee — have turned to work done with a handful of people almost 20 years ago.
Turning to silicon
Back in 2004, researchers connected a blood-filtering cartridge used in conventional dialysis to a device lined with renal cells grown from tissue of donated kidneys that weren’t suitable for transplant1. These cells help to reclaim water and electrolytes such as sodium and potassium, and help to control the production of cytokines — components of the immune system. The work, which was intended for people with traumatic kidney injury rather than chronic disease, never progressed to full clinical trials. But Fissell and Roy thought it could serve as the basis for a device to replace failed kidneys.
To recreate the filtering function of the kidney, the researchers constructed a silicon membrane with the same lithography techniques used to make computer chips. The silicon filter is more efficient than the polymer membranes conventionally used in dialysis, Roy says. At about 400 nanometres thick, it’s thin enough that the body’s own blood pressure can drive blood through it, so there’s no need for an external pump and the associated power source. The silicon filter also has narrow slits, instead of the roughly cylindrical holes of polymer membranes. The cylindrical holes can vary in shape, size and placement but the slits’ size and placement can be made more uniform. That gives the filter better selectivity, so it can pass urea molecules for elimination but retain blood cells. The silicon is also coated in materials, such as polyethylene glycol, that prevent proteins from accumulating on the surface or blood clots from forming.
Attached to the filter is a bioreactor, lined with kidney cells. The cells perform physiological functions such as transporting sodium back into the blood and shunting off excess water and toxins towards the bladder to produce urine. And because the pores in the filter are too small to allow immune cells to enter the bioreactor, the device can be used without fear of it coming under immune attack. That gives it an advantage over kidney transplants, which require people to take immune-suppressing drugs.
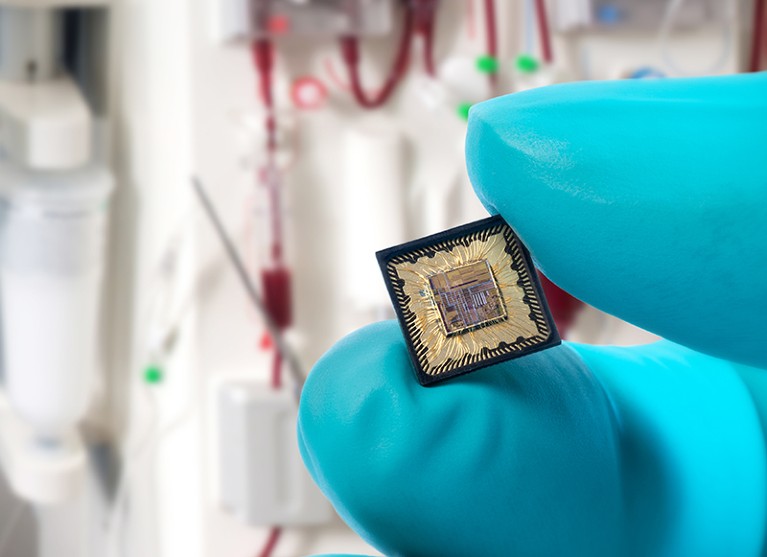
Researchers have designed a chip containing an oscillator that shakes loose certain toxins from proteins that normal dialysis does not remove.Credit: IMEC
The device is an early prototype2, but Roy hopes to develop a version that will last seven to ten years. That’s shorter than a transplanted kidney, which can last between 10 and 25 years, but in the same range as a pacemaker battery. But given the every-other-day schedule of dialysis, he says, some people might be willing to settle for an even shorter lifetime of the device.
The silicon industry’s miniaturization of electronics can help researchers to realize implantable artificial kidneys, says Fokko Wieringa, a biomedical engineer at a division of the Interuniversity Microelectronics Centre (IMEC), a microelectronics research company, in Eindhoven, the Netherlands. Wieringa, who collaborates with the Dutch Kidney Foundation in Bussum, the Netherlands, is working on components that other researchers could integrate into their designs, such as pressure sensors that can flow through an artificial kidney and monitor for obstructions.
Another innovation is a device to remove toxins that conventional dialysis cannot remove. Some toxins adhere to blood plasma proteins and therefore cannot pass through the filters. Wieringa and his colleagues designed a system on a chip that uses radio waves to disrupt the electrostatic force that causes the toxins to cling to the proteins, shaking them loose and allowing them to pass into the urine. Wieringa says the device, a miniaturization of a system built at Aachen University in Germany, would improve any sort of dialysis, including current machines, portable devices and implants.
Keep filtering and carry on
Instead of aiming for an implantable device, some researchers are focused on portable external dialysis, which might be a nearer-term solution. Jonathan Himmelfarb, a nephrologist at the University of Washington School of Medicine in Seattle, and Buddy Ratner, a chemical engineer and bioengineer at the university’s Center for Dialysis Innovation, are developing an artificial kidney that recycles dialysate — the liquid that removes urea and adds back electrolytes — instead of using litres of water to flush it away. The device is outfitted with a regeneration module, through which dialysate flows after picking up urea from the blood. Inside, ultraviolet light breaks down the absorbed urea into nitrogen and carbon dioxide, which then vent into the air before the dialysate is returned for reuse.
The device could weigh about 14 kilograms, Himmelfarb says — light enough that people could take it with them to the office or on a trip. “We’re envisioning longer, slower dialysis treatments, perhaps even continuous, like the kidneys work,” he says. That could place less strain on the body. “If we or others can really, truly make mobile forms of dialysis that don’t need connection to an external water system, it’s going to free patients up,” he says.
More from Nature Outlooks
Similarly, Ira Kurtz, a nephrologist at the University of California, Los Angeles, is working with the US Kidney Research Corporation in Roseville, California, on a suitcase-sized device that could be placed on a desk at work or beside the bed while a person sleeps; future iterations might be small enough to be wearable or even implantable. His device uses levels of filtration as well as other actions to more closely replicate the function of a biological kidney without relying on living cells. Kurtz prefers to avoid living cells because he doesn’t think that the science is advanced enough to replicate the many cell types in a kidney.
Kurtz’s device, which he is developing in collaboration with researchers3 at the University of Arkansas in Fayetteville, starts with an ultrafiltration module that contains a cellulose-based membrane. That membrane, Kurtz says, filters much more volume per surface area than do existing polymer membranes; it allows water, urea and electrolytes to pass through but blocks blood cells and proteins. The flow then reaches a nanofilter, with much smaller pores, that blocks the passage of glucose molecules that need to stay in the bloodstream. Beyond that are deionization modules, which use electrical charges characteristic of each molecule to control the movement of ions, including sodium, potassium, chloride and calcium — removing excess but allowing necessary amounts to remain in the blood. Finally, a reverse-osmosis module transports water from the synthetic urine back into the blood. This ensures that the amount of water coming out of the body matches the amount being drunk — this is either pre-programmed on the basis of average consumption or selected by the person using the device.
The researchers developing all these devices hope that theirs will be ready for animal trials in a year or two, followed by clinical trials in four or five years. Finding sufficient funding to move the projects along, however, remains a struggle. And that’s why Stacewicz is spreading the word, to increase interest and drum up funding. These days, he has home dialysis, which takes longer but is less exhausting than in-centre treatment. He and his wife have even taken road trips, although that entails wrestling a 34-kilogram machine into their car and having 35 boxes of liquid dialysate (a week’s worth) delivered to wherever they’re going. “My life is constrained now, because I’m on dialysis,” he says. “I still have dietary restraints, liquid restraints, everything else, even though doing it at home is better.”
He doesn’t expect to receive an artificial kidney, but he hopes that his advocacy will make it a reality for people in the future. “It probably won’t benefit me,” he says. “But I would like to see other people have the opportunity to live a more normal life and not have to spend years waiting for a kidney transplant.”